|
ZHEJIANG MEDICINES&HEALTH PRODUCTS I/E CO.,LTD
|
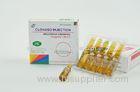
50mg / 2mL Ranitidine Injection For Gastro - Intestinal Tract / Duodenal Ulcer Treatment
| Place of Origin: | Guangdong, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
Ranitidine Injection Medicines 50mg / 2mL gastro-intestinal tract 2*5 AMPULES / BOX
<span style="
Ranitidine Injection Medicines 50mg / 2mL gastro-intestinal tract 2*5 AMPULES / BOX
RANITIDINE 50mg/2mL INJECTION
COMPOSITION:
Each 2 mL ampoule contains ranitidine hydrochloride equivalent to ranitidine 50 mg.
PHARMACOLOGICAL ACTION:
Ranitidine is a selective and competitive histamine H2-receptor antagonist. It does not exhibit anti-serotonergic or histamine H1-receptor blocking activities. Ranitidine inhibits basal and stimulated secretion of gastric acid, reducing both the volume of secretions, the acid and pepsin content but does not affect gastric mucous secretion.
The elimination half-life of ranitidine is approximately 2-3 hours.
Ranitidine is excreted via the kidneys in the free and metabolised forms. Its major metabolite is the N-oxide with smaller quantities of S-oxide and desmethyl ranitidine.
INDICATIONS:
Ranitidine is indicated for the treatment of duodenal ulcer, benign gastric ulcer, reflux oesophagitis and Zollinger-Ellison syndrome.
Ranitidine may also be used as pre-medication prior to anaesthesia in order to reduce the volume and acid content of gastric secretion, thereby minimising the consequence of the acid aspiration syndrome.
CONTRA-INDICATIONS:
Ranitidine is contra-indicated in patients known to have hypersensitivity to any of the ingredients.
Ranitidine should be avoided in patients with a history of porphyria.
DOSAGE AND DIRECTIONS FOR USE:
For intravenous or intramuscular injection.
Adults: Ranitidine may be given either as a slow (over two minutes) intravenous injection of 50 mg, diluted to
a volume of 20 mL which may be repeated every 6-8 hours, or as an intravenous infusion at a rate of 25 mg per hour for two hours; the infusion may be repeated at 6-8 hour intervals.
For pre-medication prior to anaesthesia in order to reduce the volume and acid content of gastric secretion, Ranitidine should be given intramuscularly or by a slow intravenous injection (over two minutes) of 50 mg diluted to a volume of 20 mL, 45-60 minutes before induction of general anaesthesia.
Children: There has been no experience with ranitidine in children.
SIDE EFFECTS AND SPECIAL PRECAUTIONS:
Blood:
• Leucopenia, thrombocytopenia, agranulocytosis, pancytopenia (occasionally with bone marrow hypoplasia
or aplasia). Incidence of these side-effects is rare.
Cardiovascular:
• Less frequent: Bradycardia, AV block.
Nervous system:
• Headache (sometimes severe), dizziness, mental confusion, depression, hallucinations (in the elderly and severely ill), involuntary motor disturbances. Incidence of these side-effects is rare.
Breast disorders:
• Less frequent: Breast symptoms and gynaecomastia in men.
Gastro-intestinal:
• Less frequent or rare: Diarrhoea, nausea, vomiting, constipation.
Hepato-biliary:
• Reversible and transient changes in liver function tests, hepatitis (hepatocellular, hepatocanalicular or both), with or without jaundice, acute pancreatitis. Incidence of these side-effects is rare.
Musculoskeletal:
• Less frequent: Arthralgia, myalgia.
Skin:
• Less frequent or rare: Skin rash, vasculitis, erythema multiforme, alopecia.
Ranitidine is excreted via the kidneys thus plasma levels of the medicine are increased and prolonged in
patients with severe renal impairment. It is therefore recommended that the dose be reduced to half the usual dose given daily as a single or divided dose. In the case of Ranitidine, it is recommended that in such patients doses of 25 mg are administered. Use of higher than recommended doses of intravenous H2-antagonists has been associated with rises in liver enzymes where treatment has been extended beyond five days.
Care should be taken to carry out periodic examinations of patients on prolonged maintenance treatment with ranitidine as a safeguard against the occurrence of unforeseeable consequences of drug treatment.
STORAGE INSTRUCTIONS:
Store below 25°C, protected from light.
Do not refrigerate.
KEEP OUT OF REACH OF CHILDREN.