|
ZHEJIANG MEDICINES&HEALTH PRODUCTS I/E CO.,LTD
|
1 Mega 5 Mega Benzylpenicilline Sodium Injection For Gram - Negative Micro - Organisms
| Place of Origin: | Guangdong, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
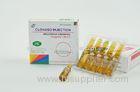
Benzylpenicilline Sodium For Injection 1Mega 5Mega Antibiotic 50VIALS / BOX
<span style="font-fam
Benzylpenicilline Sodium For Injection 1Mega 5Mega Antibiotic 50VIALS / BOX
Benzylpenicilline Sodium For Injection 1Mega
COMPOSITION:
Benzylpenicillin 600 mg per vial, as the sodium salt
PHARMACOLOGICAL ACTION:
The cell walls are essential for normal growth and development of bacteria. Peptidoglycan is the heteropolymeric component of the cell wall providing rigid mechanical stability. The action of the beta-lactam antibiotics is involved in the third stage of cell membrane cross-link formation, namely the transpeptidation reaction. The terminal glycine residue of the pentaglycine bridge is linked to the fourth residue (D-alanine) releasing the fifth residue (also D-alanine) and this step is inhibited by the beta-lactam antibiotics. The transpeptidase is probably acylated by penicillin. Various penicillin binding proteins (transpeptidases and carboxypeptidases) are associated with the bacterial cell membrane and beta-lactam antibiotics bind tightly to them. The penicillin binding proteins vary from one bacterial species to another and in their affinity for different antibiotics. The morphological changes brought about are dependant on the antibiotic, its concentration and the microbe. As the concentration is increased, growth is inhibited, bulges form and lysis follows. Resistant strains (containing no autolysins) will not lysate and different type of antibiotics are to be used.
INDICATIONS:
Benzyl penicillin is highly active against gram-positive cocci and is similar to that of penicillin V in aerobic gram-positive micro-organisms. It is five to ten times more active against gram-negative micro-organisms.
CONTRA-INDICATIONS:
Must not be administered to patients who are allergic to penicillins.
WARNINGS:
May cause death when administered to patients sensitive to penicillins. Do not add to containers of infusions containing dextrose. It may be piggy-backed via the same administration set.
DOSAGE AND DIRECTIONS FOR USE:
See table under indications. It should be limited to use by intravenous route. It can be given as an infusion over 20 to 30 minutes or by constant drip at close intervals (2 to 4 hours). Do not mix with other drugs as it is incompatible with many. Children should receive 100 000 to 250 000 units/kg per day in 4 to 6 portions. Newborns up to 1 week - 50 000 to 150 000 units/kg/day in 2 to 3 portions. Dilute with WATER FOR INJECTIONS. Use only freshly prepared solutions. Discard unused portion.
| 600 mg (1 Mega-unit) Vial | 3 g (5 Mega-unit) Vial | ||
| Concentration | mL solvent | Concentration | mL solvent |
| 100 000 units/mL | 9,6 mL | 250 000 units/ mL | 17,9 mL |
| 200 000 units/mL | 4,6 mL | 400 000 units/ mL | 10,4 mL |
| 250 000 units/mL | 3,6 mL | 500 000 units/ mL | 7,9 mL |
| 500 000 units/2 mL | 3,6 mL | 1 000 000 units/ mL | 2,9 mL |
| 1 000 000 units/mL | 0,6 mL | 2 000 000 units/5 mL | 10,4 mL |
| 1 000 000 units/5 mL | 4,6 mL | 5 000 000 units/5 mL | 2,9 mL |
| 5 000 000 units/10 mL | 7,9 mL | ||

SIDE EFFECTS AND SPECIAL PRECAUTIONS:
When administered to a hypersensitive patient, anaphylactic shock with collapse and sometimes death may occur within minutes. A generalised sensitivity reaction can occur within 1 to 3 weeks with urticaria, fever, eosinophilia, joint pains, angioneurotic oedema, erythema multiforme and exfoliative dermatitis, although an accelerated urticarial reaction can develop within hours. Glossitis, angular and aphtous stomatitis, and darkening of the tongue are liable to follow the use of penicillin.
STORAGE INSTRUCTIONS:
Store dry, below 25°C. KEEP OUT OF REACH OF CHILDREN.
Discard any unused portion.